To illustrate this, we are looking at one segment in the pharmaceutical distribution chain where goods are transported from global or regional hubs to, for example, regional distribution centers. After this point, a more finely meshed distribution network is used to transport goods typically to wholesalers within the region.
Inefficiencies in Each Step of the Monitoring Process
Two key characteristics of the distribution of goods in such a “hub-to-hub” scenario are large shipment volumes (one to many palettes) and hence large shipment value, as well as shipment lanes controlled and qualified by the pharma manufacturer. In the case of global distribution, transport also includes using air and sea freight as well as having to handle customs.
 Image 1: Overview of “hub-to-hub” distribution segment
Image 1: Overview of “hub-to-hub” distribution segment
Looking at the effort and costs involved with regards to condition monitoring within this distribution segment, we can break it up into four steps which deal with the handling of the actual data logger and its data:
- Shipment Monitoring Set-Up
- Shipment Commissioning
- Shipment Arrival
- Shipment Release
When talking about the Shipment Monitoring Set-Up, we essentially refer to preparing shipment or product profiles that reflect the condition requirements for products in terms of quality, such as temperature bands. Most solutions do not have the capability to preset these requirements, which results in additional effort when analyzing the data to determine whether or not the shipment can be released. By specifying this globally for a particular shipment or product, the profile can then be used for all future related shipments without having to reenter the information.
Now let us look at the next step on the warehouse floor – conventionally, the data logger now needs to be prepared manually, which entails entering the shipment profile for each logger, if this is even feasible, and manually linking the logger to the shipment by writing it on a label or separate list before activating the logger. Typically, this should be done by the warehouse clerk, however, as these key steps entail a high risk of human error, it is often quality management personnel who undertake this task.
We spend more money on handling the data loggers than the loggers actually cost!
Operations Lead at a big pharmaceutical company
On shipment arrival, the data needs to be retrieved manually by extracting it from the device via a computer. In most cases, the data itself is a PDF with a simple temperature curve, in other cases you can log-on to a cloud service and access the data, however, this requires an additional step and works only if you are in possession of the actual data logger itself, which functions as an authorization key. However, this is of little help if the quality manager on the sender side needs to assess and release the shipment. Hence, the PDF now needs to be sent back for assessment via email. There are even cases where the recipient needs to send the data logger back to the sender before the data can be retrieved, which increases the delay by days.
Once the quality manager has received the data to actually assess whether or not the goods can be released, the data still needs to be analyzed – in the best case an email to the recipient provides the release. In the worst case, the assessment takes longer due to the fact that only a temperature curve is provided, adding additional delays in the release of the goods. If other personnel need to be involved, long correspondences develop, which sometimes even need to be archived for legal or regulatory purposes. And what if, while preparing the data logger for shipment, a mistake in labelling or listing the logger was made? In that case, significant delays are to be expected until the goods are released.
To make matters worse, these delays, which translate into costs of people spending time on unnecessary manual tasks, lead to additional costs related to creating stock buffers requiring warehouse space as mitigation measure.
We wait for over two weeks to receive the data loggers back, so we also wait for two weeks before we can release a product and free up warehouse space for quarantined products!
Quality Assurance Lead at a big pharmaceutical company
Vaporize Monitoring Inefficiencies with MODsense
The numbers stemming from our MODsense solution being evaluated at a large pharmaceutical company, which previously has been using a conventional monitoring solution with single-use data loggers, illustrate the points mentioned above quite clearly. In this scenario, 2 data loggers per palette i.e. shipment were used in their “hub-to-hub” distribution with a data logger cost of USD 15 per logger.
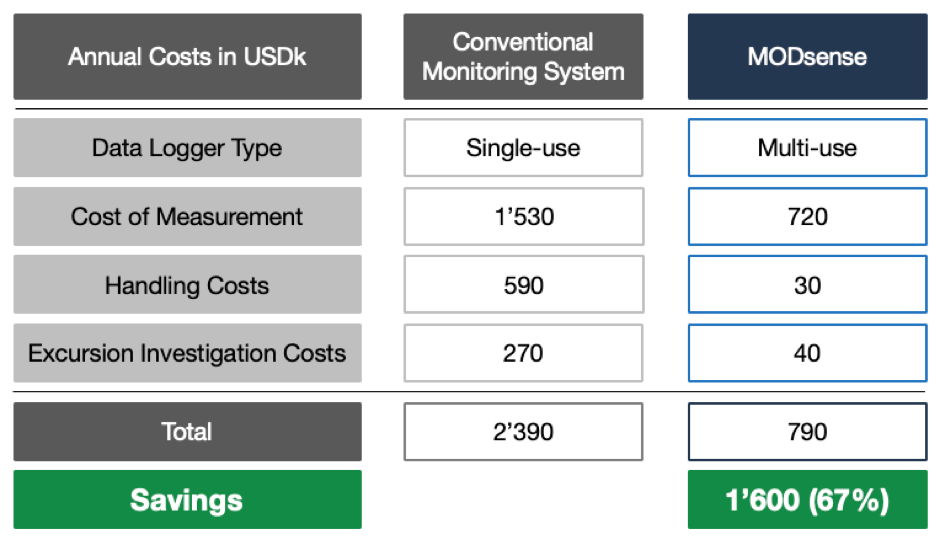 Image 2: Real-world example of cost breakdown in large pharmaceutical company
Image 2: Real-world example of cost breakdown in large pharmaceutical company
Two-thirds of the overall savings are related to effort reductions in handling as well as excursion investigations which translated into efficiency gains of factor 9. This real-world example clearly shows the significance of having a monitoring system in place which reduces the time spent on simple activities by using automation to simplify process steps and ensuring access to measurement data from anywhere on the globe immediately upon shipment arrival.
We do not stop there – MODsense can further drive efficiency gains through the integration into relevant company systems, such as the warehouse management system, ERP or product stability repository, while at the same time reducing the risk of human error further. Additionally, we provide custom analytics and reporting services to increase the value of the collected data, which provides you with more insight over your distribution process and in turn helps you to improve and further streamline activities.
Interested to learn more on how to save costs while increasing the quality of your supply chain monitoring? Then contact us today to discuss your challenges and let Modum help you succeed step by step.


