We are proud to be at a point where we are able to host such a high-profile event with an industry-wide discussion about supply chain digitalization and blockchain. This is a great achievement for Modum and I would like to again thank all of our partners, speakers and our team for having made this event possible.
I would like to use this CEO update to share the main thoughts from my opening speech at the event, with you as well: A reflection of where Modum comes from, where we are, and where we want to go in 2020 and beyond.
At the last MODday in May 2018, we demonstrated that we own the full stack of capabilities when it comes to connecting a physical shipment containing medicinal products in a supply chain to a connected IT world in order to track it over the last miles of its journey. This last stage environment provided us with a unique and invaluable learning experience about enterprise-grade requirements for high-volume processes and the corresponding cost sensitivity.
This year we went live and international and kept learning and delivering at the same fast pace. Ultimately, this brought Modum to the point where we are now ready to address the challenges of pharma supply chain tracking from end-to-end.
To show why this is relevant, I shared our view of what is happening in the industry from a manufacturer's perspective, with our MODday guests:
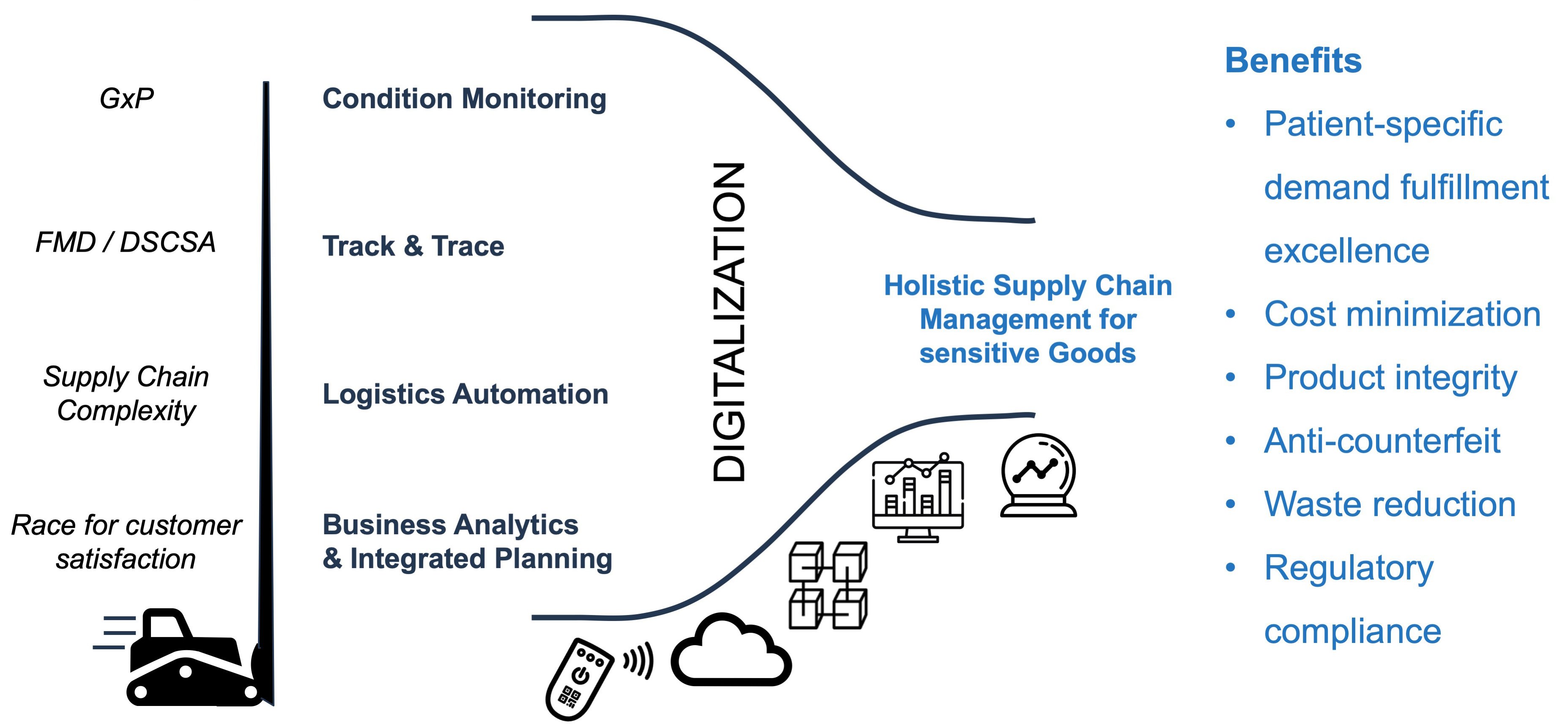
Several aspects of pharma supply chain management, such as condition monitoring, track & trace, logistics automation as well as the topic of business analytics and integrated planning are typically managed in a separate way. Each one is inevitably pushed by corresponding patient-safety, regulatory, complexity or competition drivers. Given the progressing implementation of serialization guidelines, these processes are now the target of a next wave of digitalization. This is enabled through modern technologies, data science and digitalization, in general this opens a funnel to merge these aspects in holistic processes and platforms. And this is highly relevant!
As Modum is in the midst of these changes and helping to facilitate this transformation, I shared the four main topics we discovered when engaging with the challenges of our customers and I highlighted the corresponding learnings we recommend considering when attempting to elevate supply chains to the next level:
1. Lack of access to trusted data prevents global off-the-shelf solutions
As pharma logistics is dealing with sensitive goods, it is a necessity to have sensors in the supply chain and this makes everything more complex. We see the industry looking for off-the-shelf solutions, but struggling. Not because it is hard to find sensor hardware but because scalable infrastructure and processes needed to collect trusted data that is coming from various sources, and sharing this with the right permissions is missing and preventing globally deployable solutions. This is why we focus on jointly redefining underlying processes and data flows first, and then introduce step by step manageable solutions.
2. It is all about a trusted execution of data sharing agreements
We find that many supply chain actors are paralyzed by the lack of regulatory guidance and trust in their supply chain ecosystem. A symptom of this are the numerous discussions about data ownership we witness. We believe data ownership is an outdated concept. What is needed instead are ways to independently assess the performance and conformity of shipment orders. Even more importantly, ways to transfer agreements about data sharing which are made as a one-to-one hand-shake to IT networks with the trust that they are immutably executed as agreed on. Every time. This is why we believe blockchain is the right technology.
3. Data is only used right when automatically impacting day-to-day processes
Many actors struggle to understand the true value of collecting data on a shipment-level. This is interesting as the pharma industry is used to doing it, but with the mindset to have evidence for audits and storing the data in a single purpose format. This is a huge lost opportunity. Some companies start to collect the data in a more flexible format to compile reports or dashboards for their experts to study. In our opinion, this is still a lost opportunity. We believe the true impact of the data we need to collect anyway, is when the loop is closed and the insights contained in this data are automatically extracted and used to optimize and automate the day-to-day logistics processes where the data is rooted from, without a human having to look at a dashboard.
4. Digitalization means not introducing digital tools for the same old processes
For many organizations it is a challenge to find the right approach to digitalization. Day by day, we experience that progress in a supply chain improvement project is quickest if you sit down with the right experts at the table and discuss how the current processes work, what the negative outcomes are and you then jointly come up with a target process that can be validated in a small setup. So, digitalization needs to be process and not tool or technology centric. We urge you not to consider your next supply chain visibility project as a tool replacement.
As all of those challenges need to be tackled jointly as a supply chain ecosystem, MODday was one of our contributions to facilitate this. In our keynotes we learned from Novartis, the Poseidon Network and SAP how they approach these changes. Our panel of industry experts then discussed the impact of these digitalization efforts.
Lastly, we were proud to show for the first time in hands-on showcases, how Modum approaches end-to-end tracking of pharmaceutical products on an article and shipment level, how we are expanding MODsense to provide real-time alert management of long-range shipments and the articles contained therein, how we digitalized the process of mapping the temperature of storage rooms into a thermal mapping as a service solution and how we provide smart packaging solutions with our partners DGP Intelsius and Eco Cool.
Sharing this was a great deal for us and we are looking forward to spearheading these new capabilities and sharing more details about the innovative solutions we presented at MODday, and the insights we gained through the great conversations we had through our blog.
The whole Modum team is looking forward to an exciting December and wishes you all the same.
– Simon Dössegger, CEO
30 November 2019
Updated 4 December, 2019

